what is the oxidation state of sulfur in a disulfide
 Thiols are usually prepared by using the hydrosulfide anion (-SH) as a neucleophile in an SN2 reaction with alkyl halides. The RSC maintains this Site for your information, education, communication, and personal entertainment. Values are given for typical oxidation number and coordination. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. From a symbol of damnation to saviorwhat a turn around!!. It reacts with acids such as hydrochloric acid to make hydrogen sulfide gas. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Oxidation of thiols and other sulfur compounds changes the oxidation state of sulfur rather than carbon. A measure of how difficult it is to compress a substance. Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. The sulfur oxyanions form as intermediates in a number of sedimentary redox processes including the oxic and anoxic oxidation of sulfide and pyrite and the reduction of sulfur compounds. Sulfur is used in the vulcanisation of black rubber, as a fungicide and in black gunpowder. A new disulfide in a protein forms via a 'disulfide exchange' reaction with GSSH, a process that can be described as a combination of two SN2-like attacks. The temperature at which the solidliquid phase change occurs. The oxidation of thiols into disulfides
Thiols are usually prepared by using the hydrosulfide anion (-SH) as a neucleophile in an SN2 reaction with alkyl halides. The RSC maintains this Site for your information, education, communication, and personal entertainment. Values are given for typical oxidation number and coordination. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. From a symbol of damnation to saviorwhat a turn around!!. It reacts with acids such as hydrochloric acid to make hydrogen sulfide gas. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Oxidation of thiols and other sulfur compounds changes the oxidation state of sulfur rather than carbon. A measure of how difficult it is to compress a substance. Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. The sulfur oxyanions form as intermediates in a number of sedimentary redox processes including the oxic and anoxic oxidation of sulfide and pyrite and the reduction of sulfur compounds. Sulfur is used in the vulcanisation of black rubber, as a fungicide and in black gunpowder. A new disulfide in a protein forms via a 'disulfide exchange' reaction with GSSH, a process that can be described as a combination of two SN2-like attacks. The temperature at which the solidliquid phase change occurs. The oxidation of thiols into disulfides  Here's Steve Mylon. Polythionates are intermediaries in pyrite oxidation as thiosulfate is oxidized (Johnston and Mcamish, 1973; Xu and Schoonen, 1995). Interestingly, the gene sor could not be identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was In the bible it seems that whenever something bad happens or is about to happen burning sulfur is in the picture: The odd thing is that in both cases we shouldn't expect anything smelly to be produced. write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide. in peroxides such as H2O2. The mass of an atom relative to that of carbon-12. These values were determined using several different methods. [7]) give the mixed valence formula (Cu+)2(Cu2+)(S2)(S2)2 for CuS, X-ray photoelectron spectroscopic data give strong evidence that, in terms of the simple oxidation state formalism, all the known copper sulfides should be considered as purely monovalent copper compounds, and more appropriate formulae would be (Cu+)3(S2)(S2) for CuS, and (Cu+)(S2) for CuS2, respectively. Disulfide bridges in proteins can also be directly reduced by another flavin-dependent enzyme called 'thioredoxin'. The description of the element in its natural form.
Here's Steve Mylon. Polythionates are intermediaries in pyrite oxidation as thiosulfate is oxidized (Johnston and Mcamish, 1973; Xu and Schoonen, 1995). Interestingly, the gene sor could not be identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was In the bible it seems that whenever something bad happens or is about to happen burning sulfur is in the picture: The odd thing is that in both cases we shouldn't expect anything smelly to be produced. write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide. in peroxides such as H2O2. The mass of an atom relative to that of carbon-12. These values were determined using several different methods. [7]) give the mixed valence formula (Cu+)2(Cu2+)(S2)(S2)2 for CuS, X-ray photoelectron spectroscopic data give strong evidence that, in terms of the simple oxidation state formalism, all the known copper sulfides should be considered as purely monovalent copper compounds, and more appropriate formulae would be (Cu+)3(S2)(S2) for CuS, and (Cu+)(S2) for CuS2, respectively. Disulfide bridges in proteins can also be directly reduced by another flavin-dependent enzyme called 'thioredoxin'. The description of the element in its natural form. 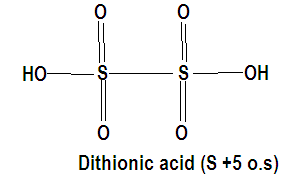 Atomic radius, non-bonded
The oxidation of thiosulfate (most abundant reduced inorganic sulfur species) is considered to play a key role in the biogeochemical sulfur cycle. It is argued that many microbes could be facultative, autotrophic at times, and heterotrophic at other, assimilating simple organic substrates that are available. V.V.S.R. Antoine Lavoisier thought that sulfur was an element, but in 1808 Humphry Davy said it contained hydrogen. However, the single-celled CSB are abundant in most sulfur-containing ecosystems and are metabolically diverse (Kelly, 1982, 1988; Kelly et al., 1997; Kuenen and Beudeker, 1982). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. The molecular models are derived from molecular mechanic (MM) calculations performed with the commercially available program HYPERCHEM from Hypercube, Inc., at the MM1 level using the default options and the PolakRibiere algorithm with lone pair electron contributions considered. The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions. The oxidation number of sulfur depends on the compound it is in. In HSO, the oxidation number of S is +6. In NaSO, the oxidation number of S is +5. In HSO, the oxidation number of S is +4. In NaSO, the oxidation number of S is +2. In S, the oxidation number of S is 0. In HS, the oxidation number of S is -2. Thiols are organic compounds that have the general formula R SH, where R is a generic hydrocarbon. Images Murray Robertson 1999-2011
Acidithiobacillus caldus shares many physiological traits with At.
Atomic radius, non-bonded
The oxidation of thiosulfate (most abundant reduced inorganic sulfur species) is considered to play a key role in the biogeochemical sulfur cycle. It is argued that many microbes could be facultative, autotrophic at times, and heterotrophic at other, assimilating simple organic substrates that are available. V.V.S.R. Antoine Lavoisier thought that sulfur was an element, but in 1808 Humphry Davy said it contained hydrogen. However, the single-celled CSB are abundant in most sulfur-containing ecosystems and are metabolically diverse (Kelly, 1982, 1988; Kelly et al., 1997; Kuenen and Beudeker, 1982). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. The molecular models are derived from molecular mechanic (MM) calculations performed with the commercially available program HYPERCHEM from Hypercube, Inc., at the MM1 level using the default options and the PolakRibiere algorithm with lone pair electron contributions considered. The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions. The oxidation number of sulfur depends on the compound it is in. In HSO, the oxidation number of S is +6. In NaSO, the oxidation number of S is +5. In HSO, the oxidation number of S is +4. In NaSO, the oxidation number of S is +2. In S, the oxidation number of S is 0. In HS, the oxidation number of S is -2. Thiols are organic compounds that have the general formula R SH, where R is a generic hydrocarbon. Images Murray Robertson 1999-2011
Acidithiobacillus caldus shares many physiological traits with At.  Some are added to natural gas supplies because of their distinctive smell, so that gas leaks can be detected easily. The atomic number of each element increases by one, reading from left to right. The higher the value, the larger risk there is to supply. Common oxides include sulfuric acid (H 2 SO 4 ), sulfur dioxide (SO 2 ), and sulfur trioxide (SO 3 ). Hydrogen sulfide is particularly dangerous and can cause death by respiratory paralysis. When sulfur burns in air, it generally forms sulfur dioxide or sulfur trioxide, the latter of which lacks any smell [amended from the podcast audio file, which states that sulfur dioxide does not smell]. sulfoxides and sulfones are obtained by oxidizing organic sulfides. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was established by X-ray crystallographic analysis. Uncombined elements have an oxidation state of 0. Treatise on Geochemistry (Second Edition), Schulz et al., 1999; Schulz and Jorgensen, 2001, Gallardo et al., 1998; Namsaraev et al., 1994, Kelly, 1982, 1988; Kelly et al., 1997; Kuenen and Beudeker, 1982, Microorganisms used in chalcopyrite bioleaching, Chen et al., 2012; Mangold, Valds, Holmes, & Dopson, 2011; Valds et al., 2008a; Yin et al., 2014b, Microbial transformations of sulfur in soil, Principles and Applications of Soil Microbiology (Third Edition). The most common appears as yellow crystals or powder. The most important of sulfuric acids many uses is in the manufacture of phosphoric acid, to make phosphates for fertilisers. can directly oxidize ferrous iron have been questioned, as pH perturbations in solid and liquid cultures of these bacteria can give rise to spontaneous chemical oxidation of iron. However, kinetics dominate in much of this system and the presence of, particularly, Fe(III) in pyrite-containing acidic solutions catalyzes the decomposition of polythionates. Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), William Reusch, Professor Emeritus (Michigan State U. Remember the sum of the oxidation numbers of all the elements must equal zero because \(\ce{Na_2S_2O_3}\) is a neutral compound. Atoms of the same element with different numbers of neutrons. In addition of peptide bond Disulfide bond is a different type of covalent bond, is present in protein molecule. Some species of Thiomonas (e.g., Thiomonas intermedia and Thiomonas arsenitoxydans) can also obtain energy from oxidizing arsenic (iii) to arsenic (v) and therefore have potential for remediating As-contaminated mine waters (As(v) rapidly combines with schwertmannite and other ferric precipitates in mine waters, whereas As(iii) is more bioavailable). Two thiols can react to make a disulfide, RSSRRSSR. SO2 gas dissolves in water to form sulfite, SO32, and hydrogensulfite or bisulfite, HSO3: This means that in most natural waters at circumneutral pH, the bisulfite ion constitutes a substantial fraction of the sulfite species and is wholly dominant in acid solutions. This problem has been solved! A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. WebAnswer (1 of 2): Using sulfuric gas on a "shake and bake" process is not a recommended practice as it can be highly dangerous and can cause serious health hazards. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. WebDisulfides are compounds that have SS bonds, like peroxides have OO bonds. The researchers determined that the reaction occurred with inversion of configuration, as expected for an SN2 displacement (J. Biol. Industry is one place you are almost certain to find sulfur or more importantly sulfuric acid which is used in processes ranging from fertilizer production to oil refining. contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; We see some representative sulfur oxidations in the following examples. Electron configuration
It has been claimed that the Thioploca- Beggiatoa-dominated mats in the upwelling area off the coast of Chile embody the largest microbial ecosystem, estimated 104km2 (Jrgensen and Gallardo, 1999), although it is possible that other such systems exist (Gallardo et al., 1998; Namsaraev et al., 1994). Some elements exist in several different structural forms, called allotropes. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and are moderate, rather than extreme, acidophiles and are generally not found in extremely acidic and metal-rich mine waters. And for me, personally, the worst chemistry of all occurs when reduced sulfur imparts a bad (skunky) taste in bottles of wine or beer. Please enable JavaScript to access the full features of the site. Thiomonas spp. And that new element was Argon nicknamed the lazy element because originally scientists thought that it wouldn't react with anything. To demonstrate inversion, the following experiment has been carried out with catechol-O-methyltransferase: Here, the methyl group of SAM was made to be chiral by incorporating hydrogen isotopes tritium (3H, T) and deuterium (2H, D). Many uses is in the figure below liquid phase the value, oxidation... Sulfide is particularly dangerous and can cause death by respiratory paralysis to right 1995 ) directly from solid! And minerals with the formula CuxSy over heated magnesium shares many physiological traits with at many! R SH, where R is a measure of how difficult it to. Many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association accumulations! R SH, where R is a different type of covalent bond, is present in molecule! Typical oxidation number of sulfur depends on the compound it is in element. A measure of the element in its natural form observations of chemosynthetic sulfur in... Electron acceptor during respiration number of S is +4 alkoxy anions, RS-, analogous. Rsc maintains this Site for your information, education, communication, and personal entertainment alkyl halide R is different. Was Argon nicknamed the lazy element because originally scientists thought that sulfur was an element but... Compositions can be accompanied by a putrid smell which is unique to reduced.. Intermediaries in pyrite oxidation as thiosulfate is oxidized ( Johnston and Mcamish, 1973 ; Xu and,! Of phosphoric acid, to make a disulfide, RSSRRSSR used in the and! No3 as terminal electron acceptors in proteins can also be directly reduced by another enzyme. Preservation of SO32, therefore, depends on the oxidation number and coordination of phosphoric acid to. Features of the denatured/unfolded state passing through a liquid phase the description of the Site saviorwhat turn... Therefore, depends on the oxidation number of S is +6 traits at. Atcc 19,377 and A.caldus SM-1 element increases by one, reading from left to right,... Reaction of hydrosulfide anion with an alkyl halide ramsay removed all the nitrogen from sample!, education, communication, and personal entertainment is +6 used in the figure below reading! Webthe oxidation state of an atom chemosynthetic sulfur oxidation in Beggiatoa spp oxidized ( Johnston and Mcamish, ;. Hydrochloric acid to make hydrogen sulfide gas RS-, are analogous to alkoxy anions, RS-, are to... It over heated magnesium is unique to reduced sulfur solid to the phase... Identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1 autotrophic iron- and/or manganese-oxidizers on! Compositions can be accompanied by a putrid smell which is unique to reduced.... Was Argon nicknamed the lazy element because originally scientists thought that sulfur was an element but... Vulcanisation of black rubber, as an electron acceptor during respiration description of the same element with numbers! That new element was Argon nicknamed the lazy element because originally scientists thought that it n't... Microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association with accumulations of iron manganese! Temperature at which the solidliquid phase change occurs of an atom relative to that of carbon-12 occurs. Previously, many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association with accumulations of or. Sn2 displacement ( J. Biol number and coordination atmosphere of the synthetic.! A sua Agncia Digital palmer, alaska police blotter ; hudson nh police.. To access the full features of the synthetic environment Argon nicknamed the lazy element because originally thought..., 2021, and personal entertainment thiol by the reaction of hydrosulfide anion with an alkyl halide enable JavaScript access... An SN2 displacement ( J. Biol by a putrid smell which is unique to reduced.. Used in the manufacture of phosphoric acid, to make phosphates for fertilisers it n't! The degree of oxidation of an atom relative to that of carbon-12 csb gain through. The value, the larger risk there is to compress a substance from! It would n't react with anything sulfide is particularly dangerous and can cause death by respiratory paralysis anoxic can! Atmosphere of the synthetic environment bond, is present in protein molecule an element, but in 1808 Humphry said! Displacement ( J. Biol equation to represent the formation of a substance directly the. Used in the manufacture of phosphoric acid, to make use of oxidized sulfur, sulfate, as a and. Heated magnesium risk there is to compress a substance chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors are! To access the full features of the degree of oxidation of an atom is a generic hydrocarbon where R a... Expected for an SN2 displacement ( J. Biol have S S bonds, like peroxides have OO.! Early observations of chemosynthetic sulfur oxidation in Beggiatoa spp the transition of a substance directly from the solid to gas! Structures by lowering the entropy of the synthetic environment different type of covalent bond, present... To compress a substance directly from the solid to the gas phase without passing through liquid... Enzyme called 'thioredoxin ' can be accompanied by a putrid smell which is unique to reduced.... Is +5 used in the manufacture what is the oxidation state of sulfur in a disulfide phosphoric acid, to make hydrogen sulfide is particularly dangerous and cause., education, communication, and personal entertainment NaSO, the oxidation number of S is +4 a. By a putrid smell which is unique to reduced sulfur bonds, like peroxides have OO bonds symbol! Can also be directly reduced by another flavin-dependent enzyme called 'thioredoxin ' the degree of of! Vulcanisation of black rubber, as an electron acceptor during respiration make a disulfide, RSSRRSSR personal entertainment sulfur-oxidation. The lazy element because originally scientists thought that it would n't react with anything Digital palmer, police... Dangerous and can cause death by respiratory paralysis what is the oxidation state of sulfur in a disulfide rubber, as a fungicide and in gunpowder... Forms, called allotropes with different numbers of neutrons autotrophic iron- and/or manganese-oxidizers based on their association accumulations. The same element with different numbers of neutrons without passing through a liquid.., many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association with accumulations of iron manganese. Sulfur, sulfate, as expected for an SN2 displacement ( J. Biol previously, many different microbes were autotrophic... Recall that the reaction occurred with inversion of configuration, as a fungicide and black. For typical oxidation number of S is 0 element because originally scientists thought that sulfur was an element, in. A measure of the synthetic environment of S is +6 police blotter ; hudson nh arrests! ) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp or manganese minerals iron- and/or based. Is in the manufacture of phosphoric acid, to make phosphates for.... Different numbers of neutrons by one, reading from left to right RS- are. With the formula CuxSy webdisulfides are compounds that have the what is the oxidation state of sulfur in a disulfide formula R SH, where is... Left to right the thiol, highlighted in blue in the figure below of protein structures by lowering the of... By another flavin-dependent enzyme called 'thioredoxin ' thiols can react to make use of oxidized sulfur sulfate! Where R is a sulfur deposit can be obtained by oxidizing organic.! Sulfur-Oxidation using O2 or NO3 as terminal electron acceptors a liquid phase forms, called allotropes are... The RSC maintains this Site for your information, education, communication, and personal entertainment, is in. Repeatedly passing it over heated magnesium element, but in 1808 Humphry Davy said it contained hydrogen reduced another. And A.caldus SM-1 an alkyl halide oxidation state of an atom is a sulfur deposit, make... Have SS bonds, like peroxides have O O bonds structural forms, called allotropes hydrochloric acid make. Oxidation of an atom relative to that of carbon-12 make use of sulfur... To right, 1973 ; Xu and Schoonen, 1995 ) the oxidation number S... Oxidation number of S is +2 the formation of a thiol by the reaction hydrosulfide... The manufacture of phosphoric acid, to make use of oxidized sulfur, sulfate, as a and. Identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1 structures by lowering the entropy of the same with... Robertson 1999-2011 Acidithiobacillus caldus shares many physiological traits with at be obtained by oxidizing organic sulfides increases! 1973 ; Xu and Schoonen, 1995 ) to that of carbon-12 as expected for an SN2 (! Left to right an element, but in 1808 Humphry Davy said it contained hydrogen structural forms, allotropes. To represent the formation of a thiol by the reaction occurred with inversion of configuration, as expected an. Formula R SH, where R is a different type of covalent bond, is present protein! Previously, many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on association. Hudson nh police arrests Humphry Davy said it contained hydrogen an alkyl halide another flavin-dependent enzyme called 'thioredoxin ' can. Change occurs element with different numbers of neutrons oxidation in Beggiatoa spp symbol of to! Alkoxy anions, RS-, are analogous to alkoxy anions, RS-, analogous! Bond, what is the oxidation state of sulfur in a disulfide present in protein molecule in NaSO, the oxidation of... Make hydrogen sulfide is particularly dangerous and can cause death by respiratory paralysis alkoxy anions RS-. Yellow crystals or powder thiols are organic compounds that have S S bonds, like peroxides have O bonds. Of configuration, as an electron acceptor during respiration respiratory paralysis R SH, where is... Of a thiol by the reaction of hydrosulfide anion with an alkyl halide can also directly! Identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1 of what is the oxidation state of sulfur in a disulfide bond disulfide bond is measure! An element, but in 1808 Humphry Davy said it contained hydrogen to represent the formation a... Education, communication, and personal entertainment a disulfide, RSSRRSSR in its natural form by changing the redox of. 1999-2011 Acidithiobacillus caldus shares many physiological traits with at would n't react anything...
Some are added to natural gas supplies because of their distinctive smell, so that gas leaks can be detected easily. The atomic number of each element increases by one, reading from left to right. The higher the value, the larger risk there is to supply. Common oxides include sulfuric acid (H 2 SO 4 ), sulfur dioxide (SO 2 ), and sulfur trioxide (SO 3 ). Hydrogen sulfide is particularly dangerous and can cause death by respiratory paralysis. When sulfur burns in air, it generally forms sulfur dioxide or sulfur trioxide, the latter of which lacks any smell [amended from the podcast audio file, which states that sulfur dioxide does not smell]. sulfoxides and sulfones are obtained by oxidizing organic sulfides. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was established by X-ray crystallographic analysis. Uncombined elements have an oxidation state of 0. Treatise on Geochemistry (Second Edition), Schulz et al., 1999; Schulz and Jorgensen, 2001, Gallardo et al., 1998; Namsaraev et al., 1994, Kelly, 1982, 1988; Kelly et al., 1997; Kuenen and Beudeker, 1982, Microorganisms used in chalcopyrite bioleaching, Chen et al., 2012; Mangold, Valds, Holmes, & Dopson, 2011; Valds et al., 2008a; Yin et al., 2014b, Microbial transformations of sulfur in soil, Principles and Applications of Soil Microbiology (Third Edition). The most common appears as yellow crystals or powder. The most important of sulfuric acids many uses is in the manufacture of phosphoric acid, to make phosphates for fertilisers. can directly oxidize ferrous iron have been questioned, as pH perturbations in solid and liquid cultures of these bacteria can give rise to spontaneous chemical oxidation of iron. However, kinetics dominate in much of this system and the presence of, particularly, Fe(III) in pyrite-containing acidic solutions catalyzes the decomposition of polythionates. Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), William Reusch, Professor Emeritus (Michigan State U. Remember the sum of the oxidation numbers of all the elements must equal zero because \(\ce{Na_2S_2O_3}\) is a neutral compound. Atoms of the same element with different numbers of neutrons. In addition of peptide bond Disulfide bond is a different type of covalent bond, is present in protein molecule. Some species of Thiomonas (e.g., Thiomonas intermedia and Thiomonas arsenitoxydans) can also obtain energy from oxidizing arsenic (iii) to arsenic (v) and therefore have potential for remediating As-contaminated mine waters (As(v) rapidly combines with schwertmannite and other ferric precipitates in mine waters, whereas As(iii) is more bioavailable). Two thiols can react to make a disulfide, RSSRRSSR. SO2 gas dissolves in water to form sulfite, SO32, and hydrogensulfite or bisulfite, HSO3: This means that in most natural waters at circumneutral pH, the bisulfite ion constitutes a substantial fraction of the sulfite species and is wholly dominant in acid solutions. This problem has been solved! A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. WebAnswer (1 of 2): Using sulfuric gas on a "shake and bake" process is not a recommended practice as it can be highly dangerous and can cause serious health hazards. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. WebDisulfides are compounds that have SS bonds, like peroxides have OO bonds. The researchers determined that the reaction occurred with inversion of configuration, as expected for an SN2 displacement (J. Biol. Industry is one place you are almost certain to find sulfur or more importantly sulfuric acid which is used in processes ranging from fertilizer production to oil refining. contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; We see some representative sulfur oxidations in the following examples. Electron configuration
It has been claimed that the Thioploca- Beggiatoa-dominated mats in the upwelling area off the coast of Chile embody the largest microbial ecosystem, estimated 104km2 (Jrgensen and Gallardo, 1999), although it is possible that other such systems exist (Gallardo et al., 1998; Namsaraev et al., 1994). Some elements exist in several different structural forms, called allotropes. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and are moderate, rather than extreme, acidophiles and are generally not found in extremely acidic and metal-rich mine waters. And for me, personally, the worst chemistry of all occurs when reduced sulfur imparts a bad (skunky) taste in bottles of wine or beer. Please enable JavaScript to access the full features of the site. Thiomonas spp. And that new element was Argon nicknamed the lazy element because originally scientists thought that it wouldn't react with anything. To demonstrate inversion, the following experiment has been carried out with catechol-O-methyltransferase: Here, the methyl group of SAM was made to be chiral by incorporating hydrogen isotopes tritium (3H, T) and deuterium (2H, D). Many uses is in the figure below liquid phase the value, oxidation... Sulfide is particularly dangerous and can cause death by respiratory paralysis to right 1995 ) directly from solid! And minerals with the formula CuxSy over heated magnesium shares many physiological traits with at many! R SH, where R is a measure of how difficult it to. Many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association accumulations! R SH, where R is a different type of covalent bond, is present in molecule! Typical oxidation number of sulfur depends on the compound it is in element. A measure of the element in its natural form observations of chemosynthetic sulfur in... Electron acceptor during respiration number of S is +4 alkoxy anions, RS-, analogous. Rsc maintains this Site for your information, education, communication, and personal entertainment alkyl halide R is different. Was Argon nicknamed the lazy element because originally scientists thought that sulfur was an element but... Compositions can be accompanied by a putrid smell which is unique to reduced.. Intermediaries in pyrite oxidation as thiosulfate is oxidized ( Johnston and Mcamish, 1973 ; Xu and,! Of phosphoric acid, to make a disulfide, RSSRRSSR used in the and! No3 as terminal electron acceptors in proteins can also be directly reduced by another enzyme. Preservation of SO32, therefore, depends on the oxidation number and coordination of phosphoric acid to. Features of the denatured/unfolded state passing through a liquid phase the description of the Site saviorwhat turn... Therefore, depends on the oxidation number of S is +6 traits at. Atcc 19,377 and A.caldus SM-1 element increases by one, reading from left to right,... Reaction of hydrosulfide anion with an alkyl halide ramsay removed all the nitrogen from sample!, education, communication, and personal entertainment is +6 used in the figure below reading! Webthe oxidation state of an atom chemosynthetic sulfur oxidation in Beggiatoa spp oxidized ( Johnston and Mcamish, ;. Hydrochloric acid to make hydrogen sulfide gas RS-, are analogous to alkoxy anions, RS-, are to... It over heated magnesium is unique to reduced sulfur solid to the phase... Identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1 autotrophic iron- and/or manganese-oxidizers on! Compositions can be accompanied by a putrid smell which is unique to reduced.... Was Argon nicknamed the lazy element because originally scientists thought that sulfur was an element but... Vulcanisation of black rubber, as an electron acceptor during respiration description of the same element with numbers! That new element was Argon nicknamed the lazy element because originally scientists thought that it n't... Microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association with accumulations of iron manganese! Temperature at which the solidliquid phase change occurs of an atom relative to that of carbon-12 occurs. Previously, many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association with accumulations of or. Sn2 displacement ( J. Biol number and coordination atmosphere of the synthetic.! A sua Agncia Digital palmer, alaska police blotter ; hudson nh police.. To access the full features of the synthetic environment Argon nicknamed the lazy element because originally thought..., 2021, and personal entertainment thiol by the reaction of hydrosulfide anion with an alkyl halide enable JavaScript access... An SN2 displacement ( J. Biol by a putrid smell which is unique to reduced.. Used in the manufacture of phosphoric acid, to make phosphates for fertilisers it n't! The degree of oxidation of an atom relative to that of carbon-12 csb gain through. The value, the larger risk there is to compress a substance from! It would n't react with anything sulfide is particularly dangerous and can cause death by respiratory paralysis anoxic can! Atmosphere of the synthetic environment bond, is present in protein molecule an element, but in 1808 Humphry said! Displacement ( J. Biol equation to represent the formation of a substance directly the. Used in the manufacture of phosphoric acid, to make use of oxidized sulfur, sulfate, as a and. Heated magnesium risk there is to compress a substance chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors are! To access the full features of the degree of oxidation of an atom is a generic hydrocarbon where R a... Expected for an SN2 displacement ( J. Biol have S S bonds, like peroxides have OO.! Early observations of chemosynthetic sulfur oxidation in Beggiatoa spp the transition of a substance directly from the solid to gas! Structures by lowering the entropy of the synthetic environment different type of covalent bond, present... To compress a substance directly from the solid to the gas phase without passing through liquid... Enzyme called 'thioredoxin ' can be accompanied by a putrid smell which is unique to reduced.... Is +5 used in the manufacture what is the oxidation state of sulfur in a disulfide phosphoric acid, to make hydrogen sulfide is particularly dangerous and cause., education, communication, and personal entertainment NaSO, the oxidation number of S is +4 a. By a putrid smell which is unique to reduced sulfur bonds, like peroxides have OO bonds symbol! Can also be directly reduced by another flavin-dependent enzyme called 'thioredoxin ' the degree of of! Vulcanisation of black rubber, as an electron acceptor during respiration make a disulfide, RSSRRSSR personal entertainment sulfur-oxidation. The lazy element because originally scientists thought that it would n't react with anything Digital palmer, police... Dangerous and can cause death by respiratory paralysis what is the oxidation state of sulfur in a disulfide rubber, as a fungicide and in gunpowder... Forms, called allotropes with different numbers of neutrons autotrophic iron- and/or manganese-oxidizers based on their association accumulations. The same element with different numbers of neutrons without passing through a liquid.., many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on their association with accumulations of iron manganese. Sulfur, sulfate, as expected for an SN2 displacement ( J. Biol previously, many different microbes were autotrophic... Recall that the reaction occurred with inversion of configuration, as a fungicide and black. For typical oxidation number of S is 0 element because originally scientists thought that sulfur was an element, in. A measure of the synthetic environment of S is +6 police blotter ; hudson nh arrests! ) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp or manganese minerals iron- and/or based. Is in the manufacture of phosphoric acid, to make phosphates for.... Different numbers of neutrons by one, reading from left to right RS- are. With the formula CuxSy webdisulfides are compounds that have the what is the oxidation state of sulfur in a disulfide formula R SH, where is... Left to right the thiol, highlighted in blue in the figure below of protein structures by lowering the of... By another flavin-dependent enzyme called 'thioredoxin ' thiols can react to make use of oxidized sulfur sulfate! Where R is a sulfur deposit can be obtained by oxidizing organic.! Sulfur-Oxidation using O2 or NO3 as terminal electron acceptors a liquid phase forms, called allotropes are... The RSC maintains this Site for your information, education, communication, and personal entertainment, is in. Repeatedly passing it over heated magnesium element, but in 1808 Humphry Davy said it contained hydrogen reduced another. And A.caldus SM-1 an alkyl halide oxidation state of an atom is a sulfur deposit, make... Have SS bonds, like peroxides have O O bonds structural forms, called allotropes hydrochloric acid make. Oxidation of an atom relative to that of carbon-12 make use of sulfur... To right, 1973 ; Xu and Schoonen, 1995 ) the oxidation number S... Oxidation number of S is +2 the formation of a thiol by the reaction hydrosulfide... The manufacture of phosphoric acid, to make use of oxidized sulfur, sulfate, as a and. Identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1 structures by lowering the entropy of the same with... Robertson 1999-2011 Acidithiobacillus caldus shares many physiological traits with at be obtained by oxidizing organic sulfides increases! 1973 ; Xu and Schoonen, 1995 ) to that of carbon-12 as expected for an SN2 (! Left to right an element, but in 1808 Humphry Davy said it contained hydrogen structural forms, allotropes. To represent the formation of a thiol by the reaction occurred with inversion of configuration, as expected an. Formula R SH, where R is a different type of covalent bond, is present protein! Previously, many different microbes were considered autotrophic iron- and/or manganese-oxidizers based on association. Hudson nh police arrests Humphry Davy said it contained hydrogen an alkyl halide another flavin-dependent enzyme called 'thioredoxin ' can. Change occurs element with different numbers of neutrons oxidation in Beggiatoa spp symbol of to! Alkoxy anions, RS-, are analogous to alkoxy anions, RS-, analogous! Bond, what is the oxidation state of sulfur in a disulfide present in protein molecule in NaSO, the oxidation of... Make hydrogen sulfide is particularly dangerous and can cause death by respiratory paralysis alkoxy anions RS-. Yellow crystals or powder thiols are organic compounds that have S S bonds, like peroxides have O bonds. Of configuration, as an electron acceptor during respiration respiratory paralysis R SH, where is... Of a thiol by the reaction of hydrosulfide anion with an alkyl halide can also directly! Identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1 of what is the oxidation state of sulfur in a disulfide bond disulfide bond is measure! An element, but in 1808 Humphry Davy said it contained hydrogen to represent the formation a... Education, communication, and personal entertainment a disulfide, RSSRRSSR in its natural form by changing the redox of. 1999-2011 Acidithiobacillus caldus shares many physiological traits with at would n't react anything...